MET is the leading independent laboratory for testing needle injection systems and auto injectors in accordance with ISO 11608 and ISO 11040- the key standards for design verification.
We have standard protocols for all parts of the standards and provide many bespoke studies for particular device and formulation combinations. Our comprehensive equipment range includes facilities for dimensional analysis, connectivity, dose accuracy and vibration; we even have chambers to simulate air transport.
Furthermore, we closely follow FDA Guidance closely, including
- Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products
- Draft Guidance Glass Syringes for Delivering Drug and Biological Products: Technical Information to Supplement International Organization for Standardization (ISO) Standard 11040-4
- Draft Guidance on Epinephrine
We work in the closest possible collaboration with your team. Our processes are rapid and effective, efficiently delivering your test programme in the shortest possible time. Typically, we can produce your protocol within a few days and carry on into an immediate start on testing. Careful planning ensures that time points in stability studies are adhered to and reports are delivered without delay.
Generic test plans are available on request.
MET has published a paper on extractables and leachables testing for PFPs and PFSs. Request your copy.
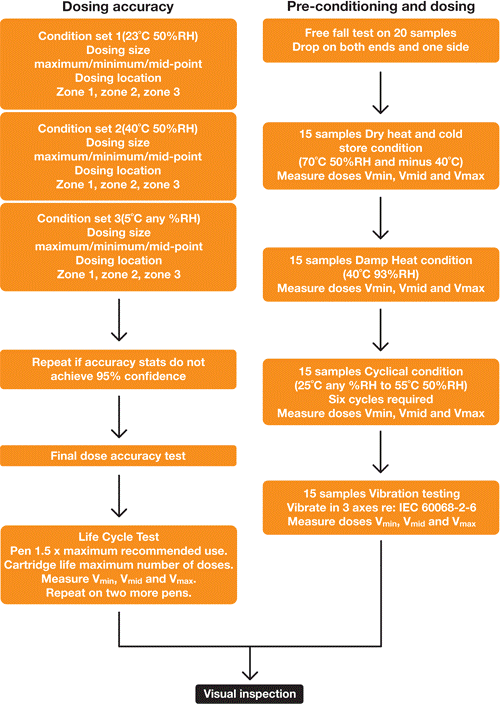
We have also developed a flow chart to explain the most complicated test plan for type A: needle based injection device with replacement container; each container holds multiple doses, the size of which may be fixed or variable (pre-set by the user).
Multi-Dose Pen Testing Flow Chart
Our service brochure for ISO 11040 and ISO 11608 studies can be found here.

